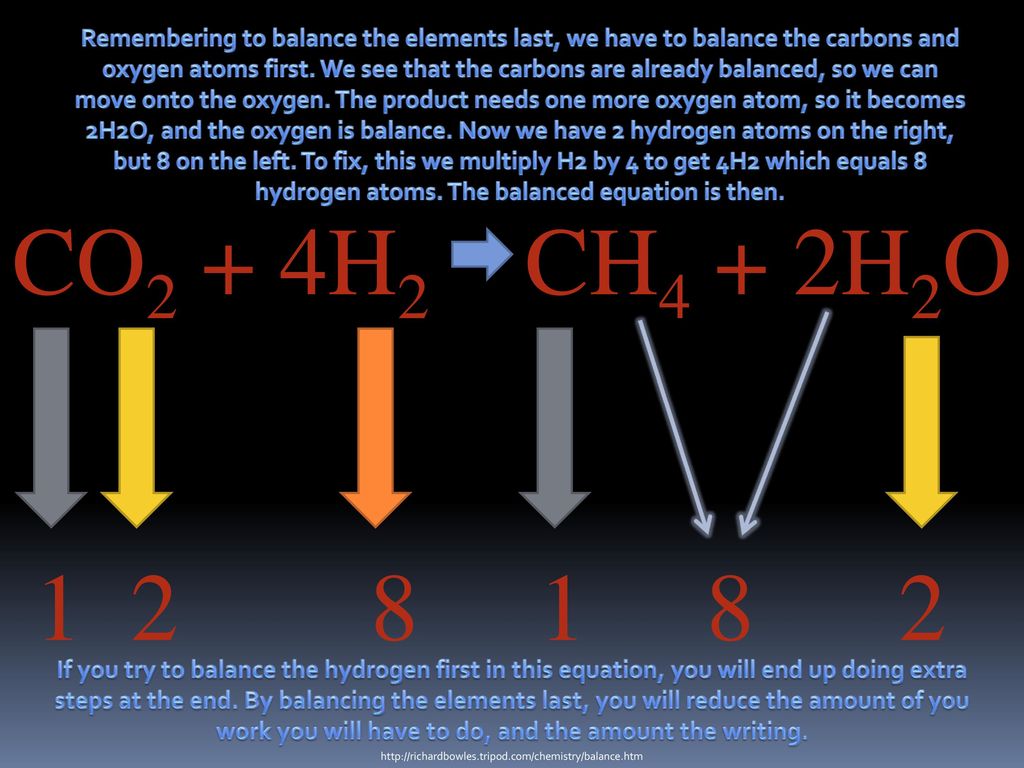
Why Do Atoms Need To Be Balanced . This is important because the law of conservation of mass states that matter cannot be created or destroyed, so the number of atoms of each element must remain the same during a chemical reaction. It is important to note that only. Balanced chemical equations have the same number and type. we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. the coefficient in a balanced equation is an idea; balancing a chemical equation means making sure that the same number of atoms of each element are present on both sides of the equation. visually understanding balancing chemical equations. to be useful, chemical equations must always be balanced. even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not. The concept of telling the.
from slideplayer.com
It is important to note that only. we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. The concept of telling the. Balanced chemical equations have the same number and type. This is important because the law of conservation of mass states that matter cannot be created or destroyed, so the number of atoms of each element must remain the same during a chemical reaction. balancing a chemical equation means making sure that the same number of atoms of each element are present on both sides of the equation. visually understanding balancing chemical equations. the coefficient in a balanced equation is an idea; to be useful, chemical equations must always be balanced. even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not.
Balancing chemical Reactions tutorial ppt download
Why Do Atoms Need To Be Balanced even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not. This is important because the law of conservation of mass states that matter cannot be created or destroyed, so the number of atoms of each element must remain the same during a chemical reaction. Balanced chemical equations have the same number and type. visually understanding balancing chemical equations. to be useful, chemical equations must always be balanced. the coefficient in a balanced equation is an idea; even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not. balancing a chemical equation means making sure that the same number of atoms of each element are present on both sides of the equation. It is important to note that only. we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. The concept of telling the.
From www.thoughtco.com
Basic Model of the Atom Atomic Theory Why Do Atoms Need To Be Balanced The concept of telling the. This is important because the law of conservation of mass states that matter cannot be created or destroyed, so the number of atoms of each element must remain the same during a chemical reaction. we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. balancing. Why Do Atoms Need To Be Balanced.
From www.slideserve.com
PPT Why do atoms form bonds? PowerPoint Presentation, free download Why Do Atoms Need To Be Balanced Balanced chemical equations have the same number and type. even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not. to be useful, chemical equations must always be balanced. The concept of telling the. we now have 2hg atoms, 2br atoms, and 4cl atoms on each. Why Do Atoms Need To Be Balanced.
From www.artofit.org
Why do atoms need to form covalent bonds Artofit Why Do Atoms Need To Be Balanced we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. to be useful, chemical equations must always be balanced. This is important because the law of conservation of mass states that matter cannot be created or destroyed, so the number of atoms of each element must remain the same during. Why Do Atoms Need To Be Balanced.
From kunduz.com
[ANSWERED] 1 Why do atoms form compounds 2 What part... Physical Why Do Atoms Need To Be Balanced we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. balancing a chemical equation means making sure that the same number of atoms of each element are present on both sides of the equation. This is important because the law of conservation of mass states that matter cannot be created. Why Do Atoms Need To Be Balanced.
From fity.club
How To Pack An Electron Application Into A Single Why Do Atoms Need To Be Balanced Balanced chemical equations have the same number and type. we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. visually understanding balancing chemical equations. This is important because the law of conservation of mass states that matter cannot be created or destroyed, so the number of atoms of each element. Why Do Atoms Need To Be Balanced.
From www.slideserve.com
PPT Chapter 7 Atoms & Bonding PowerPoint Presentation, free download Why Do Atoms Need To Be Balanced to be useful, chemical equations must always be balanced. It is important to note that only. Balanced chemical equations have the same number and type. we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. even though chemical compounds are broken up and new compounds are formed during a. Why Do Atoms Need To Be Balanced.
From slideplayer.com
Balancing Equations Mr. Shields Regents Chemistry U11 L ppt download Why Do Atoms Need To Be Balanced Balanced chemical equations have the same number and type. we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. to be useful, chemical equations must always be balanced. visually understanding balancing chemical equations. even though chemical compounds are broken up and new compounds are formed during a chemical. Why Do Atoms Need To Be Balanced.
From www.slideserve.com
PPT Chemistry and Combustion Chapter 17 PowerPoint Presentation, free Why Do Atoms Need To Be Balanced This is important because the law of conservation of mass states that matter cannot be created or destroyed, so the number of atoms of each element must remain the same during a chemical reaction. we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. The concept of telling the. balancing. Why Do Atoms Need To Be Balanced.
From learninglistlang.z19.web.core.windows.net
Balancing Chemical Equations 8th Grade Why Do Atoms Need To Be Balanced This is important because the law of conservation of mass states that matter cannot be created or destroyed, so the number of atoms of each element must remain the same during a chemical reaction. even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not. visually understanding. Why Do Atoms Need To Be Balanced.
From www.vrogue.co
Why Do Atoms Have Electrons How It Works vrogue.co Why Do Atoms Need To Be Balanced This is important because the law of conservation of mass states that matter cannot be created or destroyed, so the number of atoms of each element must remain the same during a chemical reaction. we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. even though chemical compounds are broken. Why Do Atoms Need To Be Balanced.
From aliciadesnhkaufman.blogspot.com
Which Best Explains Why Atoms Bond to Form Molecules Why Do Atoms Need To Be Balanced Balanced chemical equations have the same number and type. even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not. we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. visually understanding balancing chemical equations. This is important. Why Do Atoms Need To Be Balanced.
From www.youtube.com
Why do atoms need 8 electrons to be stable? YouTube Why Do Atoms Need To Be Balanced the coefficient in a balanced equation is an idea; It is important to note that only. The concept of telling the. to be useful, chemical equations must always be balanced. Balanced chemical equations have the same number and type. even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in. Why Do Atoms Need To Be Balanced.
From www.expii.com
Electrons — Structure & Properties Expii Why Do Atoms Need To Be Balanced we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not. balancing a chemical equation means making sure that the same number of atoms of each element are. Why Do Atoms Need To Be Balanced.
From vhmsscience.weebly.com
Chemical Equations & Conservation VISTA HEIGHTS 8TH GRADE SCIENCE Why Do Atoms Need To Be Balanced the coefficient in a balanced equation is an idea; This is important because the law of conservation of mass states that matter cannot be created or destroyed, so the number of atoms of each element must remain the same during a chemical reaction. even though chemical compounds are broken up and new compounds are formed during a chemical. Why Do Atoms Need To Be Balanced.
From www.thoughtco.com
Easy Steps for Balancing Chemical Equations Why Do Atoms Need To Be Balanced we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so balancing is complete. visually understanding balancing chemical equations. the coefficient in a balanced equation is an idea; balancing a chemical equation means making sure that the same number of atoms of each element are present on both sides of the equation.. Why Do Atoms Need To Be Balanced.
From www.artofit.org
Why do atoms need to form covalent bonds Artofit Why Do Atoms Need To Be Balanced It is important to note that only. The concept of telling the. Balanced chemical equations have the same number and type. even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not. we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and so. Why Do Atoms Need To Be Balanced.
From www.slideserve.com
PPT A QUICK BONDING REVIEW PowerPoint Presentation, free download Why Do Atoms Need To Be Balanced the coefficient in a balanced equation is an idea; balancing a chemical equation means making sure that the same number of atoms of each element are present on both sides of the equation. It is important to note that only. This is important because the law of conservation of mass states that matter cannot be created or destroyed,. Why Do Atoms Need To Be Balanced.
From solbergsphyscience.wikispaces.com
solbergsphyscience The Structure of the Atom Why Do Atoms Need To Be Balanced The concept of telling the. It is important to note that only. the coefficient in a balanced equation is an idea; even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not. we now have 2hg atoms, 2br atoms, and 4cl atoms on each side, and. Why Do Atoms Need To Be Balanced.